Prof. Dr. Alexander Groh
Institute of Physiology and Pathophysiology, Medical Faculty Heidelberg, Heidelberg University
Thalamic processing of pain and putative control by cortical feedback
What is the manifestation of pathological pain in the mammalian brain and how can we interfere with this process? Our efforts are twofold: Firstly, we aim to understand the neuronal encoding of pain in the brain. Secondly, we aim to interfere with pain-specific neuronal activity to ultimately help improving the outcome for patients suffering from pain.
Background:
Diverse types of pain all have in common that they involve sensory and emotional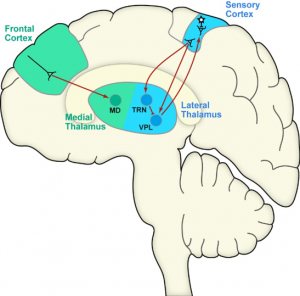 experiences. While sensory experiences fulfil important functions to protect our body from damage, emotional aspects of pain can get out of control in many chronic pain conditions. The thalamocortical system, which is essential for generating pain perceptions, contains distinct circuits for sensory and emotional aspects of pain. Lateral circuits (sensory cortex, VPL) are implicated in sensory processing and medial circuits (e.g. frontal cortex, MD) in emotional processing. This division of pain processing into distinct neuronal circuits opens new possibilities to target unwanted aspects of pain, while leaving protective functions intact.
experiences. While sensory experiences fulfil important functions to protect our body from damage, emotional aspects of pain can get out of control in many chronic pain conditions. The thalamocortical system, which is essential for generating pain perceptions, contains distinct circuits for sensory and emotional aspects of pain. Lateral circuits (sensory cortex, VPL) are implicated in sensory processing and medial circuits (e.g. frontal cortex, MD) in emotional processing. This division of pain processing into distinct neuronal circuits opens new possibilities to target unwanted aspects of pain, while leaving protective functions intact.
Current work:
We employ high-resolution recording techniques to obtain a cellular view of long-range interactions between cortical and thalamic circuits underlying nociception. By monitoring the propagation of nociceptive signals through both lateral and medial circuits, we hope to identify specific modulation points that we can optogenetically control in order to suppress nociception. We found one such modulation point in the lateral system, which consists of a small corticothalamic circuitry. Cortical layer 6 (L6) neurons project to the thalamic reticular nucleus (TRN) as well as to the ventral-posterior lateral nucleus (VPL), the principal thalamic relay nucleus of somatosensory signals en-route to the sensory cortex. Stimulation of L6 in the cortex, leads to a suppression of nociceptive responses in the VPL. We are currently investigating whether VPL suppression is mediated by feedforward inhibition from the TRN, which is under cortical L6 control.
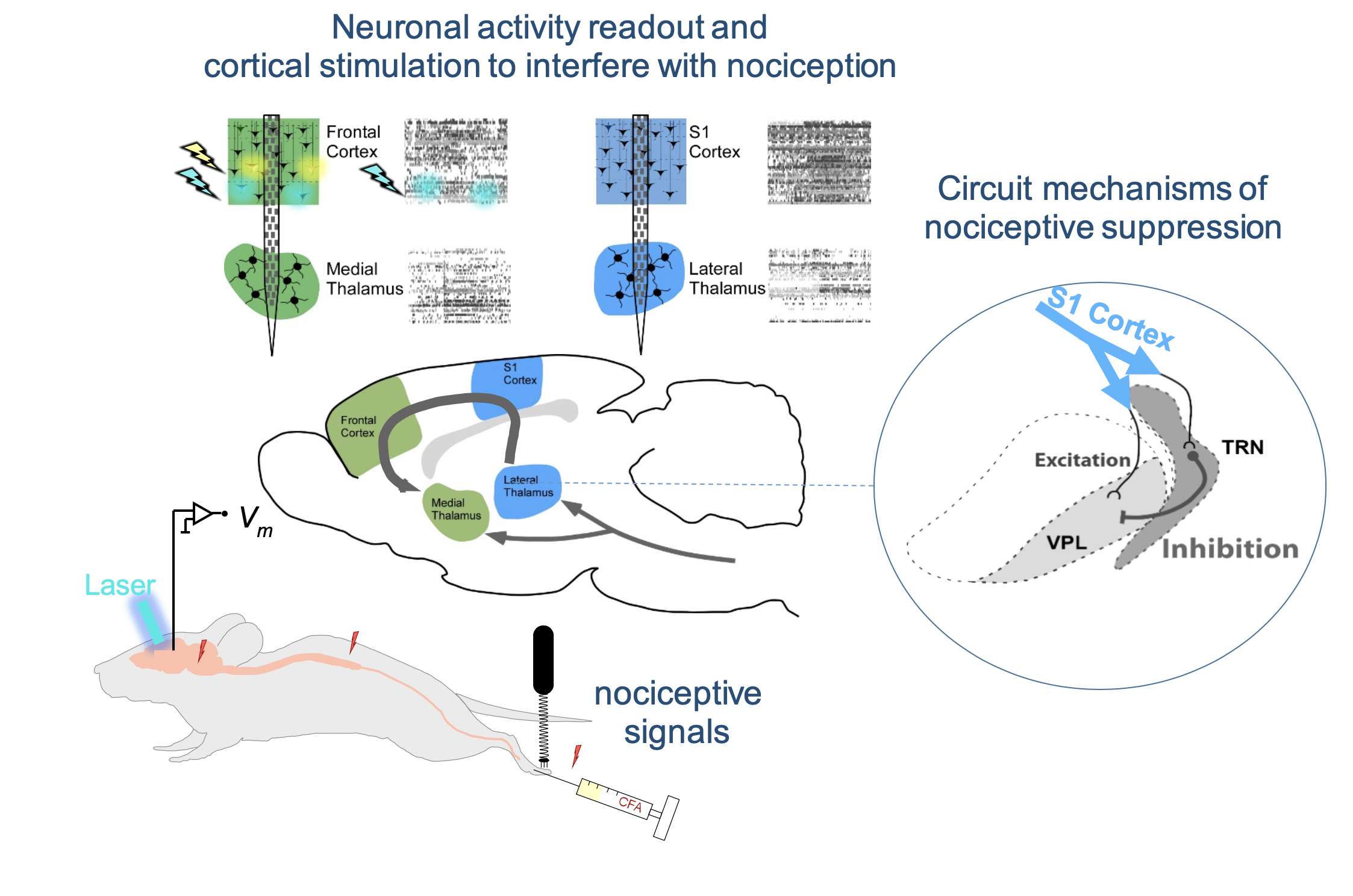
Similarly, we address the unknown function of corticothalamic pathways in the medial system. Our hypothesis is that the frontal cortex can both drive and suppress nociception in the medial thalamus via distinct corticothalamic pathways. Our goals and expected gains are to obtain a mechanistic view of nociceptive signaling in thalamocortical circuits. Our central effort is to establish and understand cortical stimulation paradigms to interfere with nociceptive signaling at the level of the thalamus. We expect that these insights will aid the translation of these strategies to be able to suppress pain very specifically.



















