Prof. Dr. Thomas Kuner
Dept. Functional Neuroanatomy, Institute for Anatomy and Cell Biology, Heidelberg University
Dr. Wolfgang Weber-Fahr
Dept. Neuroimaging, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University
Multiscale analysis of structural plasticity in cortical circuits
Background and past work:
Grey matter volume (GMV) changes during chronic pain states have initially been described in humans and are believed to be relevant to chronic pain, particularly given their reversibility upon treatment. They have recently been recapitulated in rodent models, including our own meso-scale analysis of changes in grey matter volume, connectivity and functional coupling in magnetic resonance imaging (MRI) studies on mice with neuropathic pain. However, the physical underpinnings and cellular determinants of grey matter volume changes in general, and in chronic pain states in particular, has remained elusive.
Yet, long-lasting structural changes in neocortical organization may represent a central mechanism of pain chronicity. Therefore, knowing the cellular basis of grey matter volume changes will be a major step in understanding chronic pain. During the previous funding period, we focused on subcellular changes of neuronal morphology in a mouse model of chronic neuropathic pain. In neurons of the cingulate cortex, we found changes in dendritic spine density and axonal bouton density developing on distinct timescales.
Yet, long-lasting structural changes in neocortical organization may represent a central mechanism of pain chronicity. Therefore, knowing the cellular basis of grey matter volume changes will be a major step in understanding chronic pain. During the previous funding period, we focused on subcellular changes of neuronal morphology in a mouse model of chronic neuropathic pain. In neurons of the cingulate cortex, we found changes in dendritic spine density and axonal bouton density developing on distinct timescales.
These changes were targeted to small dendritic compartments situated across the dendritic tree. We now propose to identify the input connectivity of these plastic dendritic segments. Moreover, to bridge from the subcellular level of description to mesoscopic changes reported with MRI, we propose to conduct a longitudinal multimodal investigation of structural plasticity of cortical areas in different models of chronic pain.
Hypothesis or central question for current funding round:
This project will dissect the cellular correlate of voxel-based morphometry (VBM) changes accompanying chronic pain states by comparing the number and type of cells harboured within defined neocortical volumes and by determining the physical volume occupied by a defined set of neocortical cells. It will also address the nature of white matter changes by studying axonal connectivity.
Experimental system:
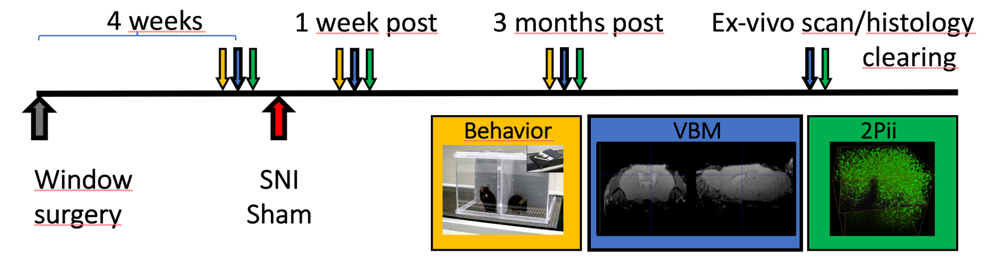
A longitudinal study design of alternating magnetic resonance imaging (MRI) and two-photon in vivo imaging (2Pii) will be employed to detect morphological changes and their cellular correlate triggered by chronic pain states in mice. Input connectivity of individual cingulate cortex neurons will be determined using a novel trans-synaptic retrograde tracing approach in conjunction with 2Pii and light sheet microscopy.
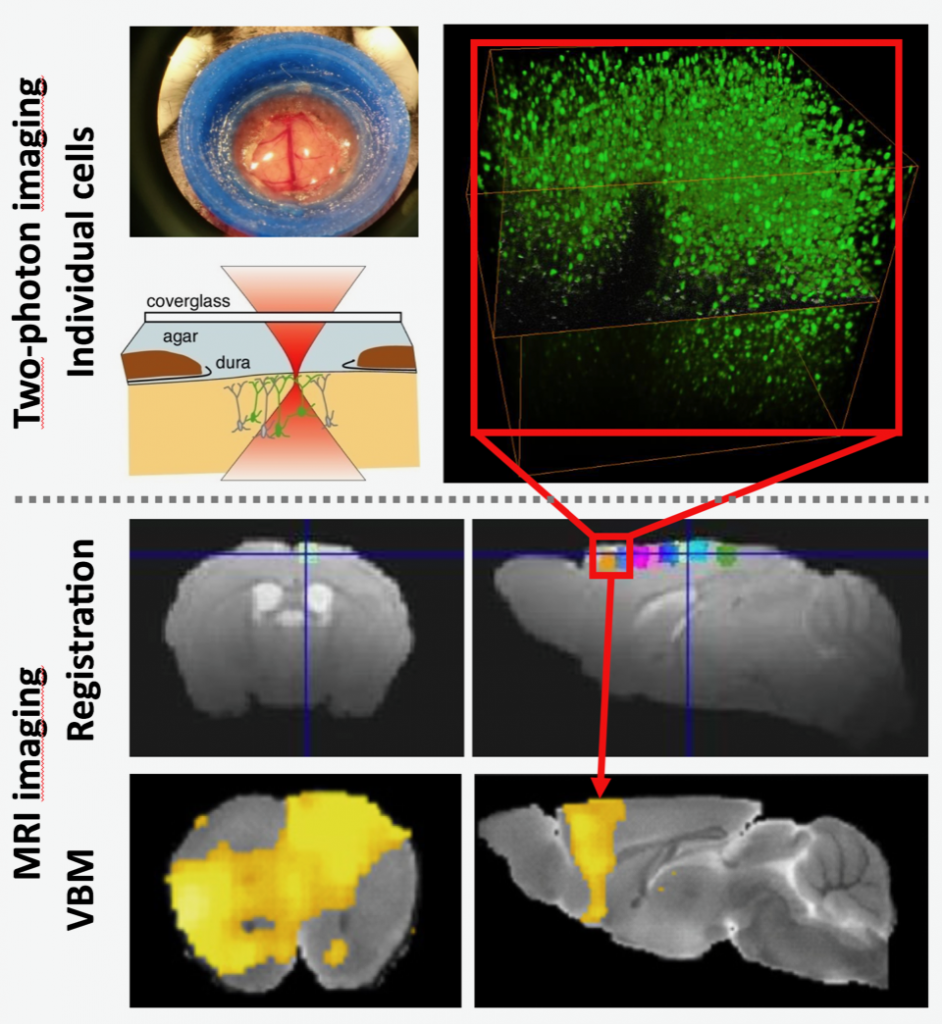
2Pii is used to image the nuclei of all cells in a cube measuring 700 µm edge length. Nuclei can be used as a proxy representing all different cell types in this volume. Thus, the number of cells and the physical distance between them can be monitored to detect changes in cell number and physical volume at different time points after nerve injury. These parameters can be correlated with changes in grey matter volume determined with VBM.
Results obtained in previous funding period:
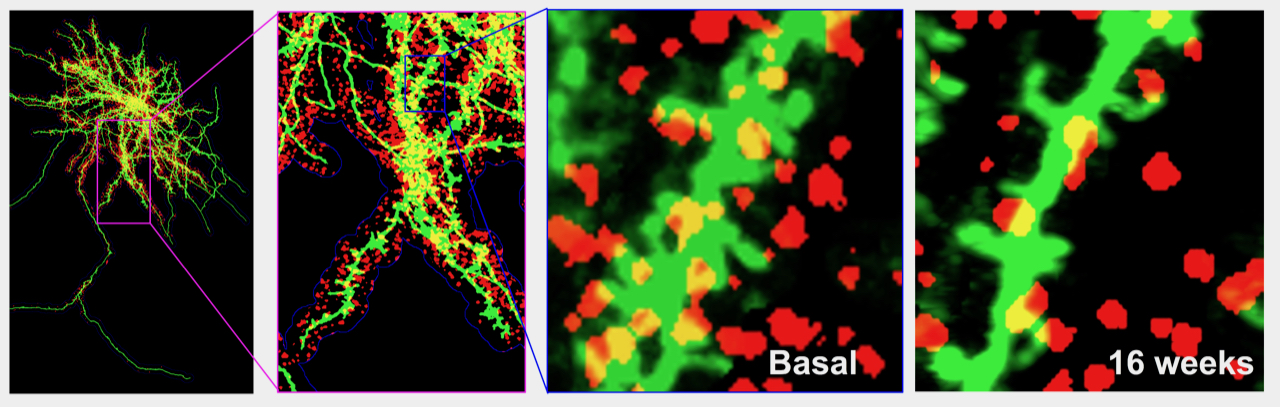 Individual cingulate neurons were imaged and changes in dendritic spines (green) and presynaptic thalamic boutons (red) were monitored over time. This example shows a decrease of both spine density and number of presynaptic boutons in response to spared nerve injury.
Individual cingulate neurons were imaged and changes in dendritic spines (green) and presynaptic thalamic boutons (red) were monitored over time. This example shows a decrease of both spine density and number of presynaptic boutons in response to spared nerve injury.



















