Prof. Dr. Norbert Weidner
Spinal Cord Injury Centre, Heidelberg University Hospital, Heidelberg University
Dr. Radhika Puttagunta
Spinal Cord Injury Centre, Heidelberg University Hospital, Heidelberg University
Functional and structural plasticity following spinal cord injury: contributions to chronic central neuropathic pain
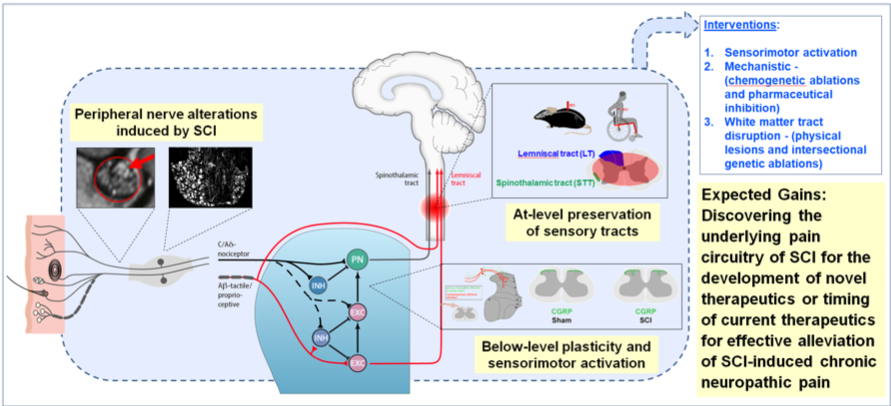
The debilitating secondary consequences of neuropathic pain after spinal cord injury (SCI) lowers the quality of patient life, restricts rehabilitative efforts and currently limited options for effective treatment are available. SCI-associated sensorimotor deprivation leads to mechanical allodynia, while sensorimotor activation has the potential to alleviate this acquired hypersensitivity. This is paralleled by maladaptive plasticity in the dorsal horn below the level of SCI. In the first funding period, we showed in contused SCI mice that treadmill training – started either acutely or delayed – specifically alleviates mechanical allodynia and spatial preference, reflecting affective measures of pain. These behavioural changes correlate with reduced sprouting of nociceptive afferents from superficial lamina into laminae III/IV, where typically non-noxious stimuli are being processed. In the second funding period, we found evidence that SCI leads to nociceptor hyperexcitability correlating with neuropathic pain. Furthermore, we observed maladaptive nociceptor plasticity in the dorsal horn, which is paralleled by pain hypersensitivity and enlargement of dorsal root ganglia (DRG) below injury level detected by MRI-based analysis, in chronic SCI subjects suffering from NP. Prophylactic administration of pregabalin and specific knockout of its targeted voltage-gated calcium channel (VGCC) α2-δ2 subunit in nociceptors effectively reduced SCI-induced NP in mouse models. Underlying mechanisms beyond presynaptic inhibition remain to be elucidated. Conversely, ablation of TrkB+ low-threshold mechanoreceptors (LTMRs) in mice and stimulation of mechanoreceptors in chronic SCI subjects failed to demonstrate a substantial effect with respect to neuropathic pain. Within the spinal cord, spinothalamic tract preservation was found to correlate with SCI-neuropathic pain in mice and individuals with SCI. Thus, hyperexcitable nociceptors within the DRG likely initiate NP, carried forward by structural rearrangements in the dorsal horn and transmitted to supraspinal levels by partially preserved spinothalamic tract projections.
This funding period we ask, can we target upstream pathways responsible for NP initiation and maintenance with pharmacological and non-pharmacological interventions in combination with the optimal time window for intervention to promote profound and sustained therapeutic effects?
Are there objectively measurable indicators above injury level predicting the likelihood of developing NP as a prerequisite for prophylactic interventions?